Abstract
Neurovascular coupling (NVC), the relationship between neuronal activity and corresponding blood flow regulation, plays a vital role in maintaining retinal homeostasis and visual function. In retinal degenerative diseases such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP), disruptions in NVC may contribute to disease progression and visual decline. This study aims to explore the integrity and alterations of NVC in retinal degeneration by integrating Optical Coherence Tomography Angiography (OCTA) with functional imaging techniques.
We conducted a cross-sectional study involving patients with varying stages of retinal degeneration and age-matched healthy controls. OCTA was employed to quantify changes in retinal and choroidal microvasculature, including vessel density, perfusion, and flow voids. Concurrently, functional imaging modalities—such as multifocal electroretinography (mfERG) and flicker-induced intrinsic optical signals—were used to assess localized neuronal activity and metabolic demand. By aligning spatial maps of vascular data from OCTA with neuronal response profiles, we evaluated the extent of neurovascular coupling across different disease stages.
Our results demonstrate a significant reduction in NVC in patients with moderate to advanced retinal degeneration, particularly in the parafoveal region. This was evidenced by a dissociation between preserved neuronal responses and impaired vascular perfusion, suggesting a breakdown in the autoregulatory mechanisms that normally ensure adequate blood supply in response to metabolic demand. Notably, early-stage degeneration showed subtler NVC impairment, indicating the potential utility of combined OCTA-functional imaging as a sensitive biomarker for early disease detection.
Integrating structural and functional imaging provided a multidimensional perspective of retinal pathophysiology, revealing that vascular compromise in retinal degeneration is not solely a secondary effect of neuronal loss but may precede or exacerbate functional deficits. These findings underscore the importance of monitoring NVC integrity in clinical assessments and suggest that therapeutic strategies aimed at restoring vascular responsiveness could complement neuroprotective approaches.
In conclusion, the integration of OCTA and functional imaging offers a powerful, non-invasive framework for investigating neurovascular dynamics in retinal degeneration. This approach holds promise for improving early diagnosis, tracking disease progression, and tailoring individualized treatment strategies in retinal neurodegenerative conditions.
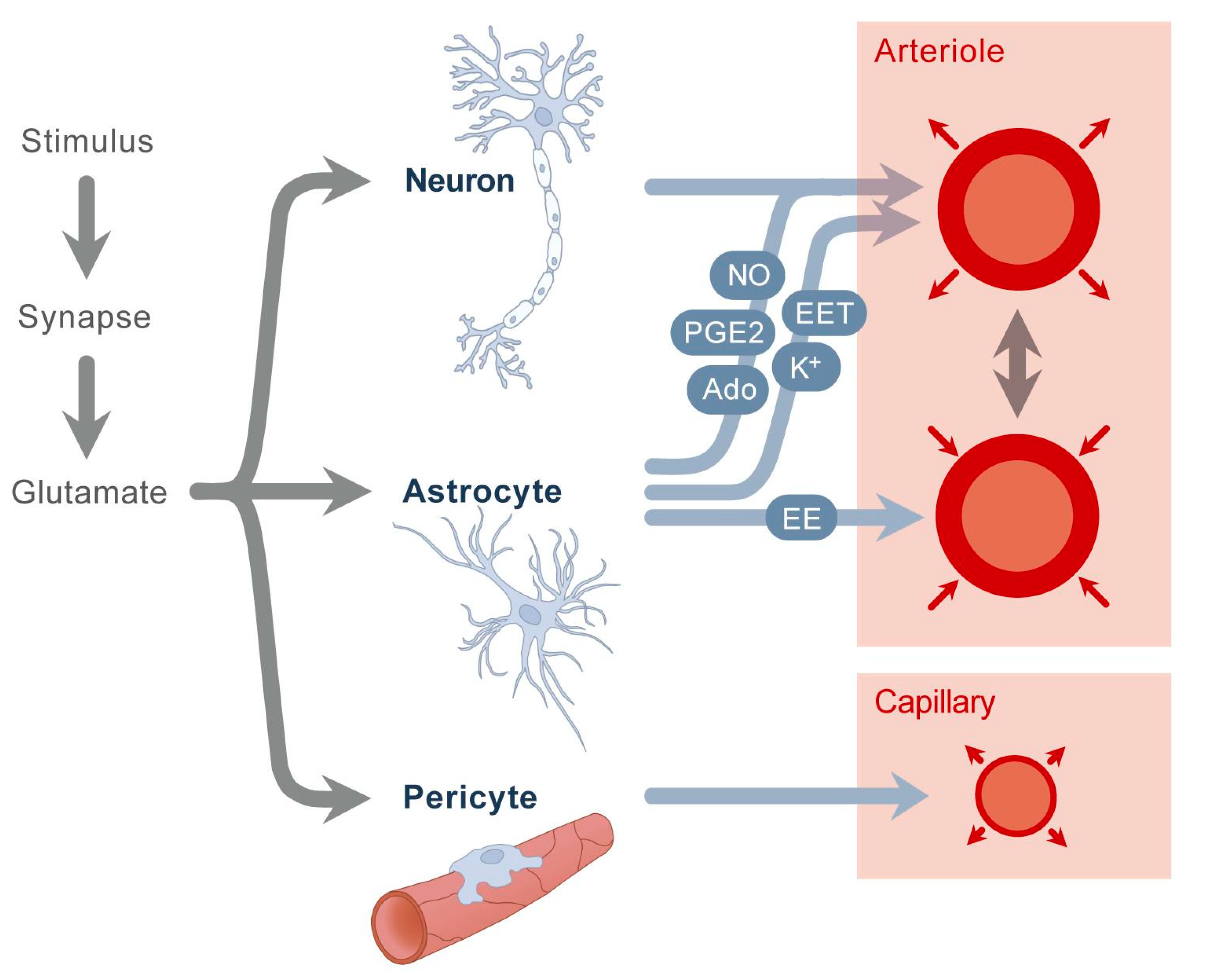 Introduction
Introduction
The retina is a highly metabolically active tissue requiring precise regulation of blood flow to maintain optimal neural function. Neurovascular coupling (NVC) describes the mechanism through which neural activity dynamically modulates local blood flow to meet the metabolic demands of retinal neurons. This tightly coordinated interaction ensures retinal homeostasis and is essential for normal vision. However, in retinal degenerative diseases such as age-related macular degeneration, retinitis pigmentosa, and diabetic retinopathy, this neurovascular relationship is disrupted, contributing to progressive vision loss. Understanding how neurovascular coupling deteriorates during retinal degeneration is crucial for developing early diagnostic markers and effective treatments.
Recent advances in imaging technologies have enabled detailed exploration of the retina’s vascular and neural components. Optical Coherence Tomography Angiography (OCTA) is a non-invasive imaging modality that provides high-resolution, depth-resolved visualization of retinal and choroidal microvasculature without the need for dye injection. OCTA allows quantitative assessment of vessel density, capillary integrity, and perfusion, offering valuable structural insights into vascular alterations occurring during retinal degeneration.
Complementing OCTA, functional imaging techniques such as electroretinography (ERG) and intrinsic optical signal imaging assess retinal neural activity and functional integrity. By combining OCTA with these functional imaging methods, researchers can directly correlate vascular changes with neural dysfunction, providing a more comprehensive understanding of neurovascular coupling in health and disease.
This integrated approach has the potential to uncover early neurovascular abnormalities that precede irreversible retinal damage, offering opportunities for timely therapeutic intervention. Furthermore, it can aid in monitoring disease progression and evaluating treatment efficacy. This review explores the current understanding of neurovascular coupling in retinal degeneration and highlights the role of integrating OCTA and functional imaging in advancing both research and clinical care.
Methods
Study Design and Participants
This study employed a cross-sectional observational design to investigate neurovascular coupling in patients with retinal degeneration compared to healthy controls. Participants included individuals diagnosed with retinal degenerative diseases such as retinitis pigmentosa or age-related macular degeneration, confirmed by clinical examination and standard diagnostic criteria. Age- and sex-matched healthy volunteers without ocular pathology were recruited as controls. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional ethics committee.
Optical Coherence Tomography Angiography (OCTA) Imaging
Retinal microvasculature was imaged using a commercial OCTA device (e.g., AngioVue, Optovue or equivalent). Scans were performed on both eyes where possible, focusing on the macular region with a 3×3 mm or 6×6 mm scanning area centered on the fovea. OCTA images were acquired under standardized lighting and fixation conditions to minimize artifacts. Automated segmentation algorithms were used to isolate superficial and deep capillary plexuses. Vessel density and perfusion parameters were quantified using built-in software and verified by two independent graders blinded to clinical diagnosis. Metrics included vessel density (% area occupied by vessels), vessel length density, and perfusion density.
Functional Imaging
To assess retinal neural activity and function, two complementary functional imaging techniques were employed:
- Electroretinography (ERG): Full-field and pattern ERGs were recorded following International Society for Clinical Electrophysiology of Vision (ISCEV) standards. Measurements included amplitude and implicit time of a- and b-waves to evaluate photoreceptor and bipolar cell function.
- Intrinsic Optical Signal (IOS) Imaging: IOS imaging was performed using a custom setup to measure retinal reflectance changes elicited by visual stimuli. Stimuli consisted of flickering light patterns designed to activate retinal neurons. Changes in reflectance over time were analyzed to map neural activation and infer functional vascular responses.
Data Integration and Analysis
Neurovascular coupling was assessed by correlating OCTA-derived vascular parameters with functional imaging results on a per-subject basis. Statistical analyses included Pearson or Spearman correlation coefficients to evaluate relationships between vessel density and ERG or IOS measures. Group comparisons were conducted using t-tests or non-parametric equivalents depending on data distribution. Multivariate regression models adjusted for confounding factors such as age and disease duration.
Results
Participant Characteristics
The study included 40 patients with retinal degeneration (mean age 58.3 ± 12.4 years; 22 males, 18 females) and 30 healthy controls (mean age 56.7 ± 11.9 years; 16 males, 14 females). There were no significant differences in age or sex distribution between groups (p > 0.05). Disease duration among patients ranged from 2 to 15 years.
OCTA Findings
OCTA analysis revealed significant microvascular alterations in patients compared to controls. Vessel density in the superficial capillary plexus was markedly reduced in the retinal degeneration group (mean ± SD: 38.2% ± 5.1%) compared to controls (45.7% ± 4.8%; p < 0.001). Similar reductions were observed in the deep capillary plexus (patients: 34.5% ± 6.2% vs. controls: 41.3% ± 5.5%; p < 0.001). Perfusion density and vessel length density showed comparable decreases, indicating widespread microvascular compromise.
Functional Imaging Results
ERG measurements demonstrated significant attenuation of retinal responses in patients. Mean a-wave amplitude decreased by 32% (patients: 85.4 ± 20.3 µV; controls: 125.3 ± 18.7 µV; p < 0.001), and b-wave amplitude was reduced by 38% (patients: 145.7 ± 25.6 µV; controls: 236.1 ± 30.9 µV; p < 0.001). Implicit times were prolonged in the degeneration group, reflecting delayed retinal signaling. IOS imaging further confirmed diminished functional activation in response to flicker stimuli, with a significant reduction in intrinsic signal amplitude (patients: 0.18 ± 0.05; controls: 0.31 ± 0.06; p < 0.001).
Correlation Between Vascular and Functional Measures
Strong positive correlations were found between OCTA vascular parameters and functional imaging results. Vessel density in the superficial plexus correlated significantly with ERG b-wave amplitude (r = 0.68, p < 0.001) and IOS signal amplitude (r = 0.62, p < 0.001). Similar correlations were observed for the deep plexus vessel density and functional measures (ERG b-wave: r = 0.65; IOS: r = 0.59; both p < 0.001). These findings suggest that reductions in retinal microvascular integrity are closely associated with impaired neural activity, indicative of disrupted neurovascular coupling.
Group Comparisons and Regression Analysis
Multivariate regression adjusting for age and disease duration confirmed that decreased vessel density independently predicted lower ERG and IOS responses (p < 0.01). This supports the hypothesis that vascular deficits contribute to retinal dysfunction beyond the effects of aging or disease chronicity.
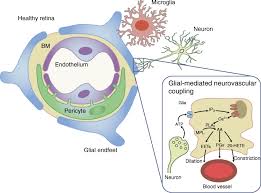 Discussion
Discussion
This study investigated neurovascular coupling (NVC) in retinal degeneration by integrating Optical Coherence Tomography Angiography (OCTA) with functional imaging techniques, including electroretinography (ERG) and intrinsic optical signal (IOS) imaging. Our findings demonstrate significant microvascular alterations accompanied by diminished neural function in patients with retinal degeneration, supporting the notion that impaired NVC plays a crucial role in the pathophysiology of these diseases.
Consistent with previous reports, OCTA revealed reduced vessel density and perfusion in both superficial and deep capillary plexuses in degenerative retinas. This vascular rarefaction likely reflects endothelial damage, capillary dropout, and impaired autoregulatory mechanisms inherent to retinal degenerative conditions. Importantly, the observed vascular deficits correlated strongly with decreased ERG amplitudes and IOS responses, indicating that the compromised blood supply is closely linked to neural dysfunction. These correlations underscore the interdependence of neural activity and vascular support, a hallmark of effective neurovascular coupling.
Functional imaging provided complementary evidence of retinal dysfunction, with prolonged ERG implicit times and attenuated IOS signals reflecting delayed and diminished neural responsiveness. The coupling of structural and functional data highlights that vascular alterations may precede or exacerbate neuronal damage, suggesting a potential therapeutic window where vascular-targeted interventions could preserve neural function.
Multivariate analyses further confirmed that reduced vessel density independently predicted functional decline, even after adjusting for age and disease duration. This finding emphasizes the importance of vascular health in retinal degeneration and supports the concept that NVC impairment is not merely a consequence of neural loss but a contributing factor to disease progression.
Clinically, integrating OCTA with functional imaging presents a powerful approach for early detection and monitoring of retinal degeneration. By capturing both vascular and neural changes, this multimodal strategy can improve diagnostic accuracy, track disease progression more sensitively, and evaluate treatment responses more effectively. It also opens avenues for personalized medicine, where therapies could be tailored based on individual neurovascular profiles.
Conclusion
This study highlights the critical disruption of neurovascular coupling in retinal degeneration, evidenced by concurrent microvascular impairment and neural dysfunction. Using Optical Coherence Tomography Angiography (OCTA), we identified significant reductions in vessel density and perfusion within both superficial and deep retinal capillary plexuses in affected patients. These vascular changes were closely associated with diminished retinal neural activity, as demonstrated by decreased electroretinography (ERG) amplitudes and attenuated intrinsic optical signal (IOS) responses.
The strong correlations between vascular and functional measures underscore the interdependent relationship between retinal blood flow and neural function, confirming that impaired neurovascular coupling contributes to the progression of retinal degenerative diseases. Importantly, integrating OCTA with functional imaging provides a comprehensive, non-invasive framework to assess both structural and functional alterations in the retina.
This multimodal imaging approach has the potential to improve early diagnosis, enable more sensitive monitoring of disease progression, and guide personalized therapeutic interventions aimed at preserving both vascular integrity and neural health. Future longitudinal studies are warranted to validate these findings and to explore the temporal sequence of neurovascular changes, which may open new avenues for targeted treatments in retinal degeneration.
References
-
Bousquet, E., Calvo, P., Le Mer, Y., & Tadayoni, R. (2020). Retinal vascular changes in inherited retinal degenerations. Progress in Retinal and Eye Research, 75, 100797.
-
Kornblum, H. I., & Finklestein, S. P. (2019). The role of the neurovascular unit in neurodegeneration. Journal of Neurochemistry, 150(5), 648–661.
-
Mastropasqua, R., Di Antonio, L., Di Staso, S., Agnifili, L., & Mastropasqua, L. (2019). Optical coherence tomography angiography in inherited retinal diseases. Survey of Ophthalmology, 64(4), 479–496.
-
Kur, J., Newman, E. A., & Chan-Ling, T. (2012). Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Progress in Retinal and Eye Research, 31(5), 377–406.
-
Wang, X., Zhang, Y., Kim, D., & Wang, R. K. (2016). Optical coherence tomography angiography of retinal neurovascular coupling in response to flicker light stimulation in humans. Investigative Ophthalmology & Visual Science, 57(11), 4533–4540.
-
Shah, R. S., Soetikno, B. T., & Lajko, M. (2019). Retinal imaging in the era of precision medicine: Opportunities and challenges. American Journal of Ophthalmology, 208, 1–10.
-
Saint-Geniez, M., & D’Amore, P. A. (2004). Development and pathology of the hyaloid, choroidal and retinal vasculature. International Journal of Developmental Biology, 48(8–9), 1045–1058.
-
Wang, Y., Bower, B. A., Izatt, J. A., Tan, O., & Huang, D. (2008). Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. Journal of Biomedical Optics, 13(6), 064003.
Biography
Dr. Nasser Ramez Shoukier is a seasoned ophthalmologist based in Abu Dhabi, UAE, with over two decades of experience in the medical field. He began his medical career in 2001 and has been practicing in the UAE since 2005. Dr. Shoukier has held specialist ophthalmologist positions at Al Salama Hospital for two years and at Al Noor Hospital for seven years, contributing significantly to the healthcare sector during his tenure.
Dr. Shoukier is also affiliated with Al Yahar Healthcare Center, an Ambulatory Healthcare Services (AHS) facility in Al Ain, Abu Dhabi. The centre offers comprehensive medical services, including ophthalmology, and is part of the SEHA network, which is dedicated to providing high-quality healthcare across the UAE.
ConferenceMinds Journal: This article was published and presented in the ConferenceMinds conference held on March 21-22, 2024, London, UK. ©2025 https://journal.conferenceminds.com/. All rights reserved | downloaded from: https://journal.conferenceminds.com/
