*Yasser El GoharyPhd, OlfatAlzaabi MD, Anshad Ummerkhan MD, Hussien Eleimy MD, Salem Deraz MD
*1,2,3,4,5Department of Pediatrics, Fujairah hospital, Emirates health services (EHS), United Arab of Emirates.
Abstract:
Gastric pneumatosis is an extremely rare condition during infancy. It has been reported in association with necrotizing enterocolitis, obstruction of the proximalgastrointestinal tract, congenital abnormalities such as pyloric stenosis. Here, we report a case of gastric pneumatosis in a full-term neonate with trisomy 13 on invasive ventilation, with ductus arteriosus dependent cyanotic congenital heart disease (CCHD) and received prostaglandin infusion. Conservative management strategies, including the use of a nasogastric tube decompression and withholding of feeding, successfully managed the gastric pneumatosis in our patient. An uneventful recovery was made after conservative management. Prompt recognition and evaluation of this condition were essential for making the diagnosis. This case describes a mature neonate treated prostaglandin who developed isolated gastric pneumatosis with evidence of pyloric obstruction.
Key Words:
Gastric pneumatosis, trisomy 13, prostaglandin, pyloric stenosis
Case Report:
A male new-born, born to 45 years old Filipino mother at 40 weeks and 3 days gestation by normal vaginal delivery. The mother was Gravida 8, Para 4 and was not following in the maternity clinic. There was premature rupture of membranes 6 hours before delivery. She was group B streptococci (GBS) positive and had history of gestational diabetes mellitus in the last pregnancy. She was on diet control.
At birth baby was hypotonic, heart rate was more than 100 per minute and there was no spontaneous breathing. The baby had cleft lip and cleft palate. The baby was intubated due to weak respiratory effort and continued invasive ventilation. Birth weight: 3.270 kg.
Saturation was not maintained even with high FIO2, so urgent echocardiography was done at 3 hour of age and diagnosed as ventricular septal defect with pulmonary atresia (VSD with PA). The pulmonary blood flow was dependant on the patent ducts arteriosus (PDA).
Prostaglandin E1 (alprostadil) infusion started at rate 0.02 mcg/kg/min then increased later to 0.03 mcg/kg/min at 3rd day of life.
On examination baby had dysmorphic features: (cleft lip and cleft Palate, Polydactyly in left hand, deformity of the fingers, Low set ears, Microphthalmia, Sandal gap between 1st and 2nd toes), Chromosomal Analysis done on 6th day of life and confirmed as a case of Trisomy 13.
In view of respiratory condition, the baby was kept on invasive mode of ventilation (Assisted control mode of ventilation with volume guarantee). Later gradually weaned and on day 11 baby was extubated to non-invasive mode of ventilation.
Trophic feeding, mainly in the form of expressed breast milk, was commenced on day 2 of life and was increased gradually according to feeding protocol and clinical status of the patient. The meconium was passed at 24 hours of life. By 5th day of life reached feeding 20 ml 3 hourly. On 7th day baby got fullness of abdomen with bloody gastric aspirate. Septic work up was done and came negative. Xray abdomen (figure 1 and 2) showed gastric pneumatosis. Ultrasound abdomen revealed distended stomach with multiple mural air densities with incomplete gastric outlet obstruction. Clinically and laboratory wise there was no features suggestive of necrotising enterocolitis.
Conservative management strategy initiated. Feeding was withheld with continuous nasogastric de-compression. Repeat echocardiography showed large PDA, so the decision was to stop prostaglandin E1 (alprostadil) infusion with careful watching of the systemic saturation. After stopping the prostaglandin E1 infusion, the systemic saturation remained around 80% which was acceptable for his cardiac condition. Serial echocardiographic follow up showed that the PDA remained patent. An uneventful recovery happened after conservative management and conformed by serial x rays (figure 3). Feeding resumed later and tolerated until reached to full feeds.
Discussion:
Gastric pneumatosis, defined as air within the wall of the stomach, is an extremely rare condition. It was first reported in a pediatric patient by the Children’s Hospital of Philadelphia in 1952 (1).
Gastric pneumatosis is primarily a radiological diagnosis (2). Isolated pneumatosis within the gastric musculature can occur in individuals of all age groups. In newborns, there are four possible circumstances in which this can develop: gastric hypoperfusion resulting from systemic hypoperfusion and critical illness (3) extension of the pneumomediastinum; mispositioned gavage tubes causing damage to the stomach mucosa (4) or proximal intestinal obstruction leading to stomach distention (5). However, in cases of necrotizing enterocolitis, generalized pneumatosis may also involve the gastric musculature (4, 5).
In 1972 Nelson summarized the possible mechanisms of the extraluminal gas collections in the gastrointestinal tract, including those of the stomach. These gas collections could either be related to infection of the visceral wall by gas-forming organisms or secondary to gastrointestinal tract obstructions. In the former case, the extraluminal gas collections were related to the direct inflammation and damage of the stomach wall. This inflammation is complicated by the diffusion of gas through breaks in the necrotic mucosa, resulting in gastric pneumatosis detected by X-ray (6).
In 2000, Taylor et al reported a neonate having this rare condition following a modified Norwood stage I operation for complex cyanotic heart disease (7). In 2006 Krueger et al reported gastric pneumatosis in a neonate with hypoplastic left heart syndrome on day 6 of life before cardiac operation (8).
In the mechanical obstruction of the proximal gastrointestinal tract, it resulted in elevated intragastric luminal pressure. These obstructions could result in an increase in diffusion through the grossly intact mucosa or the escape of the intraluminal gas through minor tears in an otherwise normal mucosa. (8). Unlike patients who develop gastric pneumatosis because of infection of the visceral wall, this latter group of patients generally enjoyed good prognosis after the prompt correction of the underlying obstruction (3).
Prostaglandin E1 (alprostadil) is widely used for maintaining the patency of ductus arteriosus in ductus-dependent congenital heart defects in neonates to improve oxygenation. Among more common side effects are fever, rash, apnea, diarrhea, jitteriness, and flushing. More severe side effects are brown fat necrosis, cortical hyperostosis, and gastric outlet obstruction, most commonly the result of antral foveolar hyperplasia or hypertrophic pyloric stenosis (12).
In the case described in this report, the cause of the formation of gastric pneumatosis remains related to use of prostaglandin. He had evidence of an upper gastrointestinal tract obstruction on the 7th day of prostaglandin E1 (alprostadil) infusion. We postulated that there could have been mild damage in the mucosa, either related to the placement of the feeding tube or secondary to the use of prostaglandin. Prostaglandin has been well known to exacerbate an upper gastrointestinal tract obstruction. Moreover, the condition may be also aggravated by invasive ventilation. An increase in intragastric pressure resulted in the submucosal dissection of air followed by the development of gastric pneumatosis detected by X-ray. This theory was also supported by the prompt resolution of the pneumatosis after conservative management and stopping the prostaglandin E1 (alprostadil) infusion. Fortunately, the PDA remained patent, which was life saving for this baby, otherwise we were planning to do urgent ductal stenting if the baby desaturates after stopping the prostaglandin E1 (alprostadil) infusion with backup cardiac surgery (Blalock-taussing shunt) if the ductal stenting failed due to any reason.
Conclusion:
Gastric pneumatosis is a rarely reported condition. In our patient, it was associated with gastric outlet obstruction secondary to the use of prostaglandin E1 (alprostadil) infusion and exacerbated with the use of invasive ventilation. Prompt recognition and evaluation of this condition were essential for making the diagnosis. Conservative management strategies, including the stoppage of prostaglandin E1 (alprostadil) infusion, use of a nasogastric tube for decompression and the withholding of feeding, successfully managed the condition in our patient.
References:
- Holgersen L O, Borns P F, Srouji M N. Isolated gastric pneumatosis. J Pediatric Surg. 1974; 9:813–816.
- Bajaj M, Ogilvy-Stuart A L. Gastric pneumatosis/interstitial emphysema of the stomach. Arch Dis Child Fetal Neonatal Ed. 2004;89:F188.
- Angadi C., Chaurasia S,, Priyadarshi M., Singh P., Basu S., Gastric pneumatosis in a neonate born late preterm on the first day of life. J Pediatr. 2023; 254: 102-103.
- Ting Y.J, Chan K.L., Wong S.C., Chim S., Wong K.Y. Gastric pneumatosis in a premature neonate. AJP Rep. 2011; 1: 11-14.
- Abusalah Z.G., George J.; Preterm baby with gastric pneumatosis: a new association with a desirable outcome. BMJ Case Rep. 2019; 12e230188.
- Nelson S W. Extraluminal gas collections due to diseases of the gastrointestinal tract. Am J Roentgenol Radium Ther Nucl Med. 1972; 115:225–248.
- Taylor D R, Tung J Y, Baffa J M, Shaffer S E, Blecker U. Gastric pneumatosis following cardiac surgery. Eur J Pediatr. 2000; 159:553–554.
- Krueger M, Ramsauer T, Dittrich S, Uhl M. Gastric pneumatosis in a neonate with hypoplastic left heart syndrome. Pediatr Radiol. 2006; 36:571.
- Bhandari V. Nasal intermittent positive pressure ventilation in the newborn: review of literature and evidence-based guidelines. J Perinatol. 2010; 30:505–512.
- Bhandari V, Finer N N, Ehrenkranz R A. et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Synchronized nasal intermittent positive-pressure ventilation and neonatal outcomes. Pediatrics. 2009; 124:517–526.
- Davis P G, Lemyre B, de Paoli A G. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev. 2001;(3):CD003212.
- Tina Perme,Senja Mali,Ivan Vidmar,Diana Gvardijančič,Robert Blumauer,David Mishaly,Iztok
Grabnar,Gregor Nemec,Stefan Grosek, PMID: 23521358,PMCID:PMC3633330,
DOI:10.3109/03009734.2013.778374.
List of Abbreviations:
CCHD: congenital cyanotic congenital heart disease.
PDA: patent ducts arteriosus.
VSD with PA: ventricular septal defect with pulmonary atresia.
List of figures:
Figure 1: X-ray chest and abdomen anterior-posterior (supine) view.
Figure 2: X-ray chest and abdomen lateral decubitus view.
Figure 3: Follow up X-ray chest and abdomen anterior-posterior (supine) view.
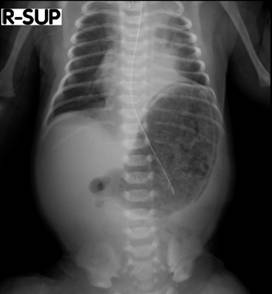
Figure 1: X-ray chest and abdomen anterior-posterior (supine) view.
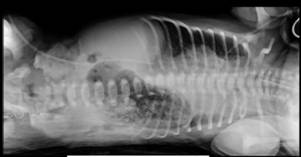
Figure 2: X-ray chest and abdomen lateral decubitus view.
Figure 1 and 2 showing: Dilated stomachand to a lesser extent dilated proximal duodendum. Distal bowel gas is present indicating an incomplete duodenal obstruction. The stomach shows peripheral cap of air surrounding its body and fundus suggestive of air leak, gastric pneumatosis.

Figure 3: Follow up X-ray chest and abdomen anterior-posterior (supine) view.
Showing: Improving gastric pneumatosis
