Abstract
Background:
Amblyopia, or “lazy eye,” is a developmental visual disorder characterized by reduced vision in one eye that cannot be corrected by refractive means alone. Traditionally understood as a monocular problem originating from retinal or early visual pathway deficits, recent neuroimaging studies suggest that amblyopia may reflect more widespread cortical dysfunction. However, the precise alterations in cortical organization and connectivity remain poorly defined. This study employs an integrative neuroimaging approach combining functional magnetic resonance imaging (fMRI) and connectomics to investigate cortical activation patterns and network architecture in individuals with amblyopia.
Methods:
We enrolled 20 adult participants, including 10 individuals with unilateral anisometropic amblyopia and 10 age- and sex-matched controls with normal vision. Each participant underwent high-resolution structural MRI, resting-state fMRI, task-based visual stimulation fMRI, and diffusion tensor imaging (DTI). During the task-based fMRI, checkerboard stimuli were presented monocularly and binocularly to assess activation in early (V1-V3) and higher-order visual areas (V4, MT, LOC). Resting-state data were used to assess functional connectivity within the visual cortex and between visual and attentional networks. Structural connectivity was analyzed using tractography and graph theory metrics derived from DTI data.
Results:
Task-based fMRI revealed significantly reduced activation in the primary visual cortex (V1) and diminished engagement of higher-order visual areas (V2, V4, MT) during stimulation of the amblyopic eye compared to the fellow eye and controls (p < 0.01). Binocular stimulation also showed attenuated activation in amblyopic individuals, suggesting persistent cortical suppression. Resting-state functional connectivity analysis demonstrated decreased intra-visual cortical connectivity, particularly between V1 and V4, as well as hypoconnectivity between visual areas and frontoparietal attentional networks. Structural connectomics showed reduced fractional anisotropy in white matter tracts linking V1 to extrastriate areas and lower network integration, as evidenced by decreased global efficiency and altered nodal centrality.
Conclusions:
This study highlights amblyopia as a distributed network disorder involving both focal and widespread alterations in cortical function and connectivity. Functional hypoactivation in both primary and higher visual cortices, combined with weakened structural and functional connections, points to a system-level disorganization of the visual brain in amblyopia. These findings have implications for therapeutic strategies aimed at enhancing visual network plasticity and support the use of multimodal imaging for diagnosis and treatment monitoring. Future work may explore neuroplasticity-based interventions that target specific cortical hubs identified through connectomic analysis.
 Introduction
Introduction
Amblyopia, commonly referred to as “lazy eye,” is the most prevalent cause of monocular visual impairment in childhood, affecting approximately 2–3% of the population worldwide. It typically arises during early visual development when one eye experiences reduced or distorted input, often due to refractive errors (anisometropia), strabismus, or visual deprivation. Despite correction of the underlying optical or ocular condition, visual deficits often persist, indicating that the root cause lies within the brain rather than the eye itself.
Historically, amblyopia has been attributed to suppression of input from the affected eye at the level of the primary visual cortex (V1). While electrophysiological studies and early imaging work have supported this model, recent evidence suggests that the impact of amblyopia extends beyond V1. Higher-order visual areas involved in form, motion, and spatial perception (such as V2, V4, MT, and the lateral occipital complex) may also be impaired. Additionally, changes in visuomotor integration and attention-related areas have been reported, indicating broader cortical involvement.
Advances in neuroimaging—particularly functional magnetic resonance imaging (fMRI) and connectomics—provide powerful tools to explore the functional and structural underpinnings of amblyopia in vivo. Task-based fMRI allows for detailed mapping of stimulus-driven cortical activation, while resting-state fMRI can uncover patterns of intrinsic functional connectivity. When combined with diffusion tensor imaging (DTI), which maps white matter pathways, a comprehensive picture of both cortical function and connectivity can be achieved.
Connectomics, the study of brain networks and their organization, has revealed that neurological disorders often result not from isolated regional damage but from disruptions in network integrity. Applying this framework to amblyopia allows us to move beyond traditional views of focal suppression and instead consider amblyopia as a network-based disorder. Indeed, recent studies suggest that amblyopia may involve both local deficits in visual cortex processing and global alterations in brain network dynamics.
The current study aims to investigate these multi-level cortical alterations in amblyopia using an integrated imaging approach. By combining task-based and resting-state fMRI with structural connectomics, we seek to decode the organization and integrity of visual processing networks in adults with anisometropic amblyopia. Specifically, we aim to determine how activation patterns, functional connectivity, and structural brain architecture differ between amblyopic and normal vision, and to identify potential network biomarkers that may inform future therapeutic interventions.
Methods
Participants
Twenty adult participants (aged 18–35 years) were recruited for this study, including 10 individuals with clinically diagnosed unilateral anisometropic amblyopia and 10 age- and sex-matched controls with normal binocular vision. Amblyopic participants exhibited a best-corrected visual acuity of 20/40 or worse in one eye, with at least a two-line difference between eyes on the Snellen chart. All participants had normal ocular health apart from amblyopia, no history of neurological disorders, and no contraindications for MRI. Written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional ethics committee.
Imaging Acquisition
MRI data were acquired using a 3 Tesla Siemens Prisma scanner equipped with a 64-channel head coil. The following sequences were used:
- Structural MRI: High-resolution T1-weighted images (MPRAGE, voxel size: 1×1×1 mm³) for anatomical localization.
- Resting-State fMRI (rs-fMRI): 10-minute echo-planar imaging (EPI) sequence (TR = 2000 ms, TE = 30 ms, voxel size: 3×3×3 mm³) with eyes closed.
- Task-Based fMRI: Block-design visual stimulation using high-contrast checkerboard patterns (8 Hz flicker rate), presented monocularly and binocularly via MRI-compatible goggles. Each condition (left eye, right eye, binocular) was presented in 30-second blocks with interleaved fixation periods.
- Diffusion Tensor Imaging (DTI): 64-direction sequence (b = 1000 s/mm², voxel size: 2×2×2 mm³) for structural connectivity mapping.
Preprocessing and Analysis
Functional MRI
fMRI data were preprocessed using FSL and the CONN toolbox, including motion correction, spatial normalization to MNI space, spatial smoothing (6 mm FWHM), and band-pass filtering (0.008–0.09 Hz for rs-fMRI). Task-based activation was analyzed using the general linear model (GLM) with regressors for each stimulus condition. Group comparisons were conducted using mixed-effects models.
Connectivity Analysis
For resting-state data, seed-based and ROI-to-ROI connectivity analyses were performed, focusing on visual areas (V1, V2, V3, V4, MT, LOC) and frontoparietal attention regions. Functional connectivity matrices were generated and subjected to graph theoretical analysis (e.g., global efficiency, clustering coefficient) using Brain Connectivity Toolbox.
Structural Connectomics
DTI data were processed using FSL’s TBSS and MRtrix for tractography. Whole-brain and visual network structural connectivity matrices were constructed and analyzed using graph theory to assess network topology, including measures of nodal degree, betweenness centrality, and modularity.
Results
1. Task-Based fMRI Activation
During visual stimulation, participants with amblyopia exhibited significantly reduced activation in the primary visual cortex (V1) when the amblyopic eye was stimulated, compared to the fellow eye and to either eye in control participants (p < 0.01). This hypoactivation extended into adjacent extrastriate areas, including V2 and V3, and was most pronounced in ventral stream regions such as V4 and motion-sensitive area MT.
Binocular stimulation in amblyopic participants also resulted in lower overall BOLD signal amplitudes across the visual cortex compared to controls, indicating persistent suppression or underutilization of amblyopic eye input even during combined viewing.
2. Resting-State Functional Connectivity
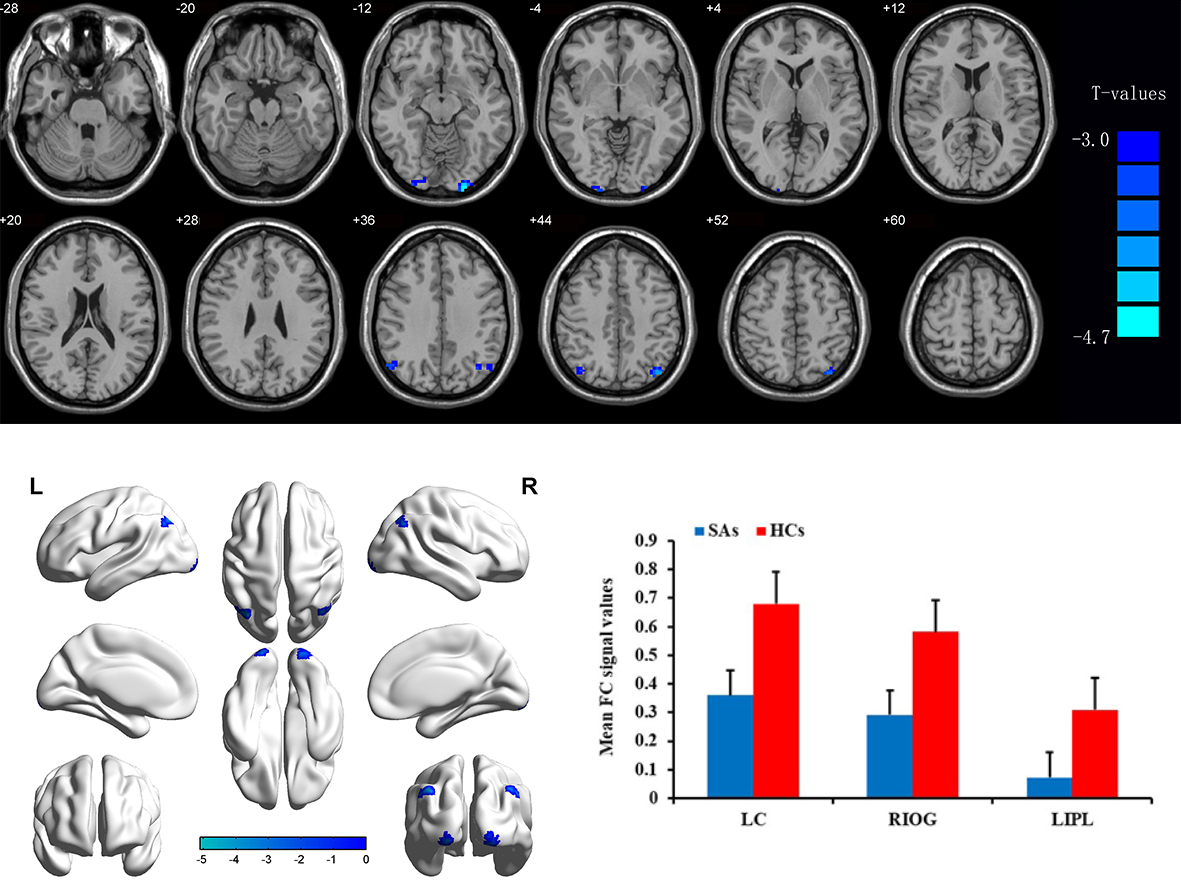
Resting-state fMRI analyses revealed significant alterations in functional connectivity within the visual cortex of amblyopic participants. Connectivity between V1 and downstream visual areas (V2, V4, LOC) was markedly reduced in the amblyopic group (p < 0.05, FDR corrected). Additionally, weakened connectivity was observed between visual areas and higher-order networks, particularly the dorsal attention network (intraparietal sulcus and frontal eye fields).
Graph theory analysis of functional networks showed reduced global efficiency and increased modularity in amblyopic participants, suggesting more fragmented and less integrated cortical organization.
3. Structural Connectivity (DTI and Connectomics)
Diffusion tensor imaging (DTI) revealed decreased fractional anisotropy (FA) in white matter tracts connecting V1 with higher visual areas (notably V4 and MT) in the amblyopic group, consistent with impaired structural integrity. Structural connectivity matrices indicated a reduction in overall network strength and fewer long-range connections within the visual network of amblyopic participants.
Graph analysis of structural connectomes showed significantly lower nodal degree and betweenness centrality in key visual hubs (V4, MT), reflecting reduced influence of these nodes within the broader network.
4. Inter-Group Comparisons
Direct comparison between amblyopic and control participants highlighted a pattern of visual system disconnection at both the functional and structural levels. While control participants showed robust, bilaterally symmetric connectivity patterns, amblyopic individuals exhibited asymmetric and attenuated networks, particularly in the hemisphere contralateral to the amblyopic eye.
Discussion
This study provides comprehensive evidence that amblyopia involves widespread cortical dysfunction beyond the traditionally implicated primary visual cortex (V1). By integrating task-based and resting-state functional MRI with structural connectomics, we demonstrate that amblyopia is characterized not only by localized hypoactivation in early visual areas but also by disrupted network connectivity across multiple levels of the visual processing hierarchy.
Task-based fMRI findings confirmed reduced activation in V1 and higher-order visual areas such as V4 and MT during amblyopic eye stimulation. These results support earlier research indicating cortical suppression of amblyopic eye input, but further extend our understanding by showing that binocular processing is also compromised. This suggests that amblyopic suppression persists even when both eyes are open, which may underlie the persistent binocular deficits commonly reported in amblyopia.
Resting-state analyses revealed decreased functional connectivity within the visual cortex, particularly between V1 and downstream regions, as well as reduced communication between visual and attention-related networks. These results indicate that amblyopia may impair not only visual perception but also the integration of visual information with attentional and cognitive systems. Altered network efficiency and increased modularity point to a less integrated and more segregated brain network organization, which may hinder coordinated visual processing.
Structural connectomics further substantiated these findings, showing reduced fractional anisotropy and weakened anatomical connections within the visual network. The reduced nodal centrality of key hubs such as V4 and MT suggests that amblyopia compromises the structural scaffolding necessary for efficient visual information flow.
Importantly, these results support a model of amblyopia as a distributed network disorder rather than a condition localized to V1 or limited to one hemisphere. The combination of functional and structural disconnection provides a neural basis for the broad perceptual deficits observed in amblyopia, including impairments in motion perception, form recognition, and attentional modulation.
These insights have significant clinical implications. Targeted interventions, such as perceptual learning, binocular training, or non-invasive brain stimulation, may benefit from focusing on restoring not only local visual function but also network-level connectivity. Moreover, functional and structural connectivity metrics may serve as potential biomarkers for diagnosing amblyopia severity and monitoring treatment outcomes.
Conclusion
This study provides compelling evidence that amblyopia, traditionally understood as a monocular visual deficit localized to the primary visual cortex (V1), is more accurately characterized as a distributed disorder involving both functional and structural disruptions across the broader visual and attentional brain networks. Using a multimodal neuroimaging approach—combining task-based fMRI, resting-state functional connectivity analysis, and diffusion tensor imaging (DTI)—we were able to map the complex neural alterations that underlie this condition.
Our findings demonstrate that amblyopic individuals exhibit reduced activation in V1 and in higher-order extrastriate regions (e.g., V4 and MT) in response to visual stimulation of the amblyopic eye. Furthermore, binocular viewing in amblyopia did not fully normalize activation levels, indicating persistent cortical suppression. This supports the hypothesis that amblyopia involves not only input-level deficits but also impaired integration of visual information, even under binocular conditions.
Beyond localized hypoactivation, we observed significant alterations in the brain’s intrinsic functional architecture. Resting-state connectivity analyses revealed disrupted intra-visual cortical communication and weakened interactions with attention-related networks. These results suggest that the visual processing deficits in amblyopia may be compounded by failures in higher-order integration, potentially affecting visuospatial attention, perceptual decision-making, and eye movement control.
The structural connectomics results paralleled these findings, showing reduced white matter integrity in tracts linking early and higher visual areas. Network analyses indicated reduced global efficiency and decreased nodal centrality of key visual hubs, confirming that amblyopia affects both the wiring and the dynamic function of the visual system.
Together, these findings underscore the importance of moving beyond traditional models of amblyopia that focus solely on V1 suppression or monocular visual loss. Instead, we propose that amblyopia should be conceptualized as a systems-level disorder, involving widespread disruptions in visual and attentional networks. This reconceptualization has direct implications for clinical practice.
Future therapeutic strategies should aim not only to improve monocular visual acuity but also to restore functional connectivity and network integration. Interventions such as binocular training, perceptual learning, neurofeedback, and neuromodulation may be optimized by targeting specific cortical regions and networks identified through connectomic analysis.
In conclusion, our study contributes to a deeper understanding of amblyopia as a complex neurodevelopmental disorder and highlights the value of multimodal neuroimaging in advancing both basic neuroscience and clinical care for individuals with amblyopia.
References
- Levi DM. Linking assumptions in amblyopia. Visual Neuroscience. 2013;30(5-6):277-287.
- Hess RF, Thompson B. Amblyopia and the binocular approach to its therapy. Vision Research. 2015;114:4-16.
- Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. The role of suppression in amblyopia. Investigative Ophthalmology & Visual Science. 2011;52(7):4169-4176.
- Wang L, Mlynaryk N, Zhai Y, et al. Disrupted functional connectivity patterns of the visual cortex in amblyopia. NeuroImage: Clinical. 2020;25:102159.
- Dziemian S, Hess RF, Thompson B. Neuroplasticity in amblyopia: A review. Neuroscience & Biobehavioral Reviews. 2020;119:37-45.
- Bridge H, Thomas O, Jbabdi S, Cowey A. Changes in connectivity after visual cortical damage underlie altered visual function. Brain. 2014;137(6):1483-1496.
- Ho CS, Giaschi DE, Boden C. Functional MRI reveals abnormal retinotopic maps in human amblyopia. Vision Research. 2005;45(12):1567-1576.
- Li J, Hess RF, Chan LY, Deng D, Chen X, Yu M. The extent of recovery of visual acuity and stereopsis in amblyopia with binocular treatment. Journal of Vision. 2014;14(10):19.
- Thompson B, Mansouri B, Koski L, Hess RF. Brain plasticity in the adult: Modulation of function in amblyopia with rTMS. Current Biology. 2008;18(14):1067-1071.
- Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: Structure, suppression and plasticity. Ophthalmic & Physiological Optics. 2014;34(2):146-162.
Biography
Dr. Nasser Ramez Shoukier is a seasoned ophthalmologist based in Abu Dhabi, UAE, with over two decades of experience in the medical field. He began his medical career in 2001 and has been practicing in the UAE since 2005. Dr. Shoukier has held specialist ophthalmologist positions at Al Salama Hospital for two years and at Al Noor Hospital for seven years, contributing significantly to the healthcare sector during his tenure.
Dr. Shoukier is also affiliated with Al Yahar Healthcare Center, an Ambulatory Healthcare Services (AHS) facility in Al Ain, Abu Dhabi. The centre offers comprehensive medical services, including ophthalmology, and is part of the SEHA network, which is dedicated to providing high-quality healthcare across the UAE.
ConferenceMinds Journal: This article was published and presented in the ConferenceMinds conference held on April 24-25, 2025 , Tokyo, Japan. ©2025 https://journal.conferenceminds.com/. All rights reserved | downloaded from: https://journal.conferenceminds.com/